NABL Accreditation Consultant
NABL Accreditation Consultant
If you are in the search of a NABL Accreditation Consultant who has years of experience NABL accreditation of laboratory, feel free to contact us anytime. National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science & Technology, Government of India, and is registered under the Societies Act. It has been established with the objective to provide Government, Industry and Society in general with a scheme for third-party assessment of the quality and technical competence of testing and calibration laboratories. We help laboratories by providing training, documentation etc. Our professionals provide NABL Accreditation Services to the clients in a non-discriminatory manner and are accessible to all testing and calibration laboratories in India and abroad as well regardless of their ownership, legal status, size and degree of independence. Our qualified consultant will carry on the project in Testing, Calibration and Medical Laboratories with the help of the NABL coordinator/Quality Manager.
National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science & Technology, Government of India, and is registered under the Societies Act. NABL has been established with the objective to provide Government, Industry and Society in general with a scheme for third-party assessment of the quality and technical competence of testing and calibration laboratories. Government of India has authorized NABL as the sole accreditation body for Testing and Calibration laboratories. In order to achieve this objective, NABL provides laboratory accreditation services to laboratories that are performing tests / calibrations in accordance with NABL criteria based on internationally accepted standard for laboratory accreditation ISO/IEC 17025. These services are offered in a non-discriminatory manner and are accessible to all testing and calibration laboratories in India and abroad, regardless of their ownership, legal status, size and degree of independence.
Project Plan :
Our qualified consultant will carry on the project in Testing, Calibration and Medical Laboratories with the help of the NABL coordinator/Quality Manager. In all quality management systems documentation is an essential component of NABL accreditation. NABL standards require various documentation. It is suggested that the Laboratory prepare an Quality manual. It must be remembered that Quality Manual is a policy document, which has to be supplemented by a set of other documents like Procedural Manuals, Work Instructions etc. to align the Quality System in accordance with NABL Criteria. The laboratory must ensure that the procedures described in the Quality Manual and other documents are being implemented. For preparing Quality Manual or verifying its contents.
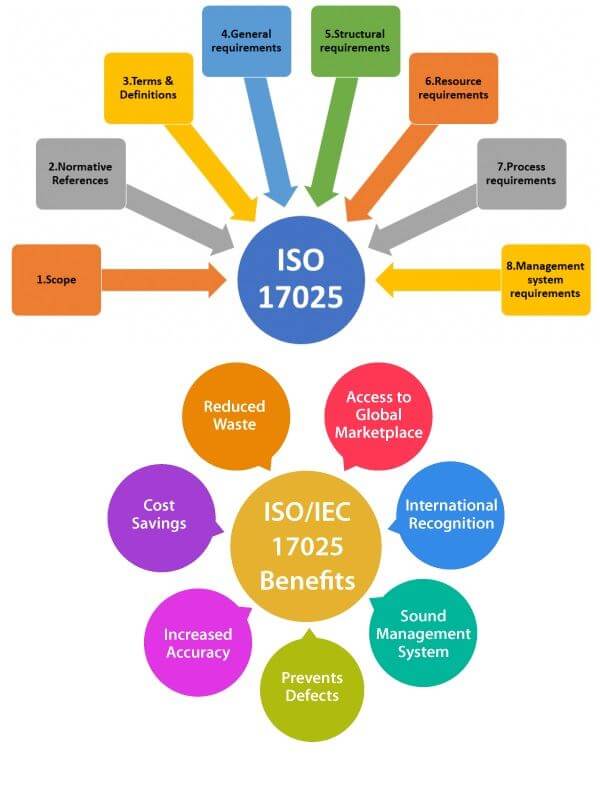
What is ISO 17025:2017 Certification
What is ISO/IEC 17025:2017 Certification
ISO/IEC 17025:2017 specifies the general requirements for the competence, impartiality and consistent operation of laboratories. Each & every laboratories have to continuously provide good service if they want to stand best in competitive marketplace. ISO 17025 is the only internationally accepted standard for laboratory quality systems that provides a globally accepted basis for accreditation.ISO/IEC 17025:2017 is applicable to all organizations performing laboratory activities, rather than number of employees.
Laboratory customers, regulatory authorities, organizations and schemes using peer-assessment, accreditation bodies, and others use ISO/IEC 17025:2017 in confirming or recognizing the competence of laboratories.
The first version (ISO Guide 25) of this standard was published in 1999, by the International Organization for Standardization (ISO).The second version was released in 2005, which is more aligned with the ISO 9001:2000 standard regarding quality system words. ISO 17025:2005 specifies the general requirements a laboratory has to meet if it is to be recognized as competent to carry out tests and/or calibrations, including sampling. It covers testing and calibration performed using standardized methods, methods not covered by standardized methods, and laboratory- developed methods. However, compliance with regulatory and safety requirements on the operation of laboratories are not covered by ISO 17025:2005.
If a laboratory wishes accreditation for part or all of its testing and calibration activities, it should select an accreditation body that operates in accordance with ISO/IEC 17011 (General requirements for accreditation bodies accrediting conformity assessment bodies)
An overview of ISO 17025:2017
ISO/IEC 17025 is the global quality standard for testing and calibration laboratories. It is the basis for accreditation from an accreditation body.
This International Standard is applicable to all organizations performing tests and/or calibrations regardless of the number of personnel or the extent of the scope of testing and/or calibration activities. These include first-, second- and third-party laboratories, and laboratories where testing and/or calibration forms part of inspection and product certification.
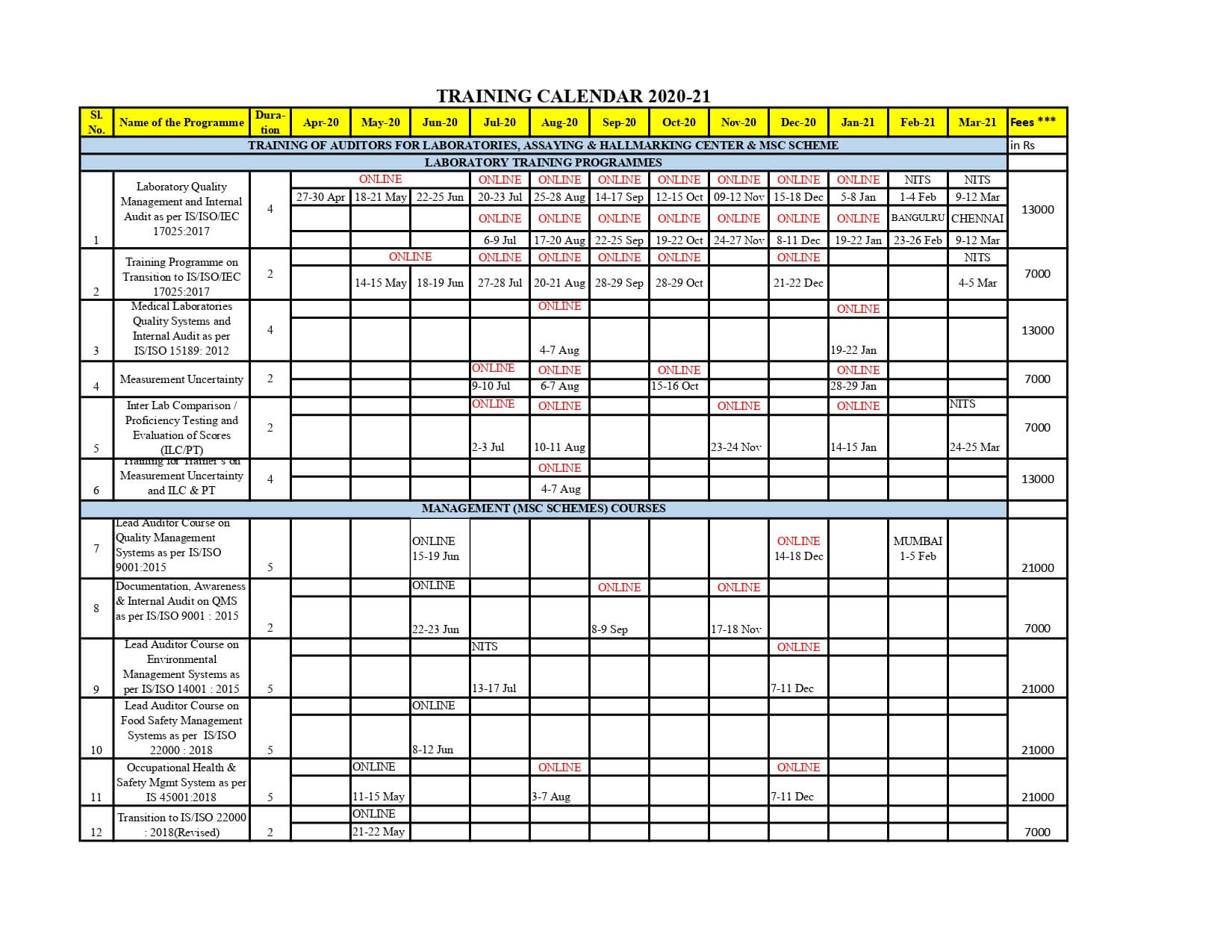
TRAINING CALENDER 2020-21 ISO/IEC 17025:2017 for Certification
Following are some of the programmes which are being conducted every month ISO/IEC 17025:2017
Lead Assessors Courses on Quality Management Systems (as per IS/ISO 9001),Environmental Management Systems (as per IS/ISO 14001),Occupational Health & Safety Management Systems (as per IS 18001), Food Safety Management Systems (as per IS/ISO 22000), etc.
Awareness programmes/ Documentation for QMS, EMS, FSMS, OHSMS, EnMS, etc and Integrated Management Systems.
Training for implementation of Service Quality by Public Service Organizations as per IS 15700.
Training for Internal Audit for QMS, EMS, FSMS and for Integrated Management Systems.
Training programmes for Laboratory Quality Management Systems (as per ISO 17025) and Medical Laboratory Quality Management Systems (as per IS/ISO 15189).
Training programmes on Measurement Uncertainty and Inter Laboratory Comparison and Proficiency Testing.
Training programme on Conformity Assessment – General requirement for proficiency testing as per ISO/IEC 17043
Training programmes for Assaying and Hallmarking CentersTraining programmes for Standards Developing Organizations and for Technical Committee members
Training programmes on Consumer Awareness/Consumer protection
Training Programmes on Certification Procedures for BIS Applicants
Training Programmes on Product Certification Marks Scheme for Licensees.
Training Programmes on Compulsory Registration Scheme(CRS) for Electronics and IT Goods for AIRS(Authorized Indian Representatives)
ISO/IEC 17025:2017 DOWNLOAD TRAINING CALENDER Pdf Format
Click here
LIST OF OFFICERS NABL TO BE CONTACTED PDF
Click here
Faq Question
Measuring instruments are the 'heartbeat' of your company as they check and measure your production processes. They control the quality of your products and in the end are responsible for the success and the profitability of your business.A regular check of your 'heartbeat' with traceable calibration equipment ensures your quality always matches the customer's expectations, which is vital where ISO 17025 certification is involved.
This calibration is done under the guidelines of a national accrediting organization to the ANSI/ISO 17025 – 2017 standard and will be delivered with documentation according to the accrediting body.
If a calibration can be performed for you, you receive a calibration certificate on which the precise results, such as calibration number, measurement data, measurement uncertainties and signatures are noted by the staff. These calibrations are extremely important in precisely reconstructing all the dimensional standards and recording the inaccuracies in detail in an official prescript.
We calibrate equipment from most major manufactures, such as HP, HPL, Fluke, Agilent, Tektronix and many others – Equipment types include : Multimeters, handheld to 8 digit bench meters, Oscilloscopes – analog and digital, Current clamps, Frequency counters, Thermometers, Wattmeters, power analyzers, Multifunction and multiproduct calibrators, Voltage, current and resistance calibrators, Process calibrators, Standard resistors, Fluke Network Test Tools, Energy Meters.
Why Us?
Over the years, we have proved our credibility in the industry and created an indelible position. The following factors illustrate why we are different from our counterparts : We have all the necessary licenses required for a Service Provider. We have important contacts that help us carry our work easily and fast.
Our highly skilled professionals are aware how to do their work efficiently and are well trained in maintaining customer relations. Keeping in mind the current market scenario, each service we provide is economical. We guarantee complete satisfaction. Click for more detail
ISO 17025
ISO 17025: Traceable Calibration'Calibration' means that the test results from a measuring device (or source) of unknown accuracy, are compared with a device whose accuracy is known. This device is universally accepted as a 'reference'. Calibrations are traceable to the International System of Units (SI) through National Metrological Institutes, ratiometric techniques and natural physical constants.Calibration records any deviation from this standard and corrects it when necessary.
Each instrument has a specific calibration procedure which indicates exactly how and what must be checked. Regular and traceable calibration combines the forces of accuracy and certainty and provides you with the key aspect of ISO 17025 registration. Click for more detail
NABL Accreditation Consultant
If you are in the search of a NABL Accreditation Consultant who has years of experience NABL accreditation of laboratory, feel free to contact us anytime. National Accreditation Board for Testing and Calibration Laboratories (NABL) is an autonomous body under the aegis of Department of Science & Technology, Government of India, and is registered under the Societies Act. It has been established with the objective to provide Government, Industry and Society in general with a scheme for third-party assessment of the quality and technical competence of testing and calibration laboratories.
We help laboratories by providing training, documentation etc. Our professionals provide NABL Accreditation Services to the clients in a non-discriminatory manner and are accessible to all testing and calibration laboratories in India and abroad as well regardless of their ownership, legal status, size and degree of independence. Our qualified consultant will carry on the project in Testing, Calibration and Medical Laboratories with the help of the NABL coordinator/Quality Manager. Click for more detail
Our Services
Welcome to our company Ruvik Automation Pvt. Ltd.
Get A Quick QuoteContact us
GET A QUICK QUOTE
Location: E-41, Galli No-3, Samaspur Road, Pandav Nagar, East Delhi,
Delhi - 110091, India - Earth
(Click for Location)